TAMPA, Fla. — Michael Anderman wanted to help bring another COVID-19 vaccine to Americans.
“Anything that I could do for my part to help everyone get out of their houses, get this world back together, I was happy to do,” Anderman said.
So, he and his daughter signed up for the Novavax vaccine trial.
“We went for the first shot, and then we went back for some more blood work then they gave us the second shot, went back for more blood work,” he said.
The University of South Florida Morsani College of Medicine was one of the sites in Tampa Bay conducting the Novavax clinical study.
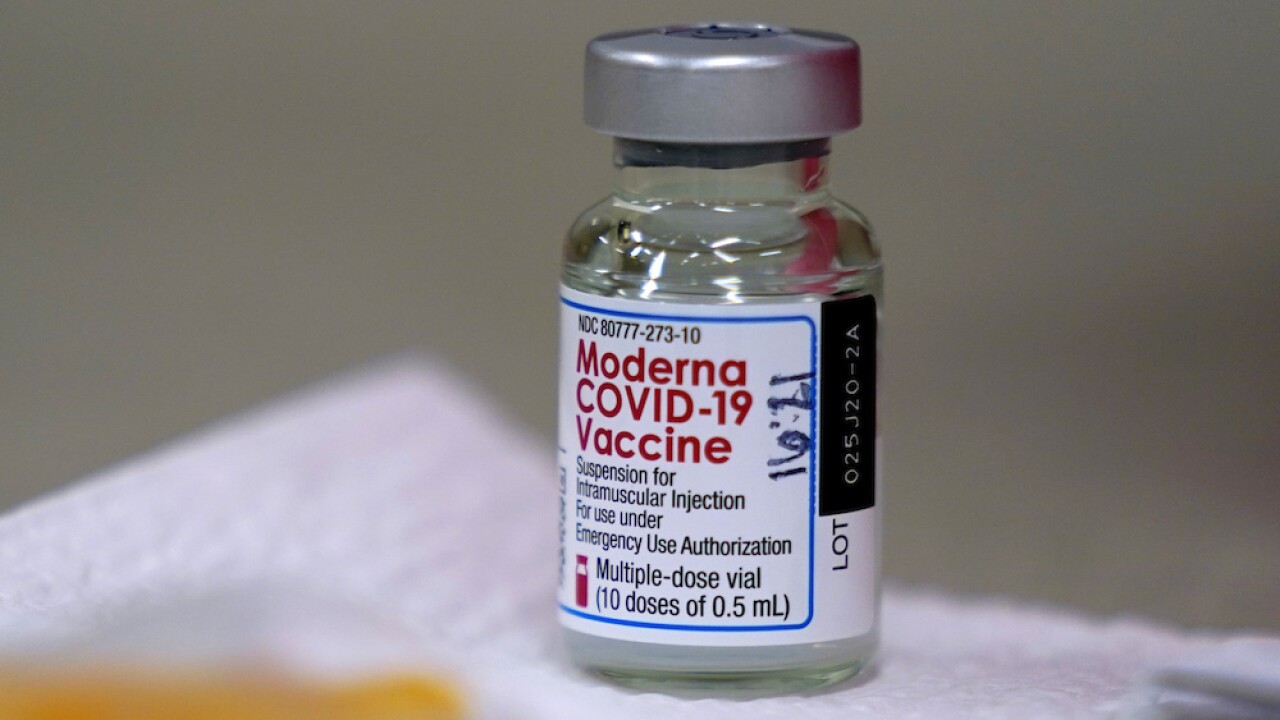
On Wednesday, USF announced it has been selected as one of the sites for clinical testing of the Moderna mRNA COVID-19 vaccine in children between six months and 11-years-old.
Moderna said it would have 12,000 pediatric patients in the United States and Canada during the phase 2/3 research trial called KidCOVE.
“Widespread vaccination is the best defense against COVID-19, and this rigorous scientific study may go a long way toward increasing vaccine access in this younger demographic,” said Charles Lockwood, MD, senior vice president of USF Health and dean of the USF Health Morsani College of Medicine.
USF said it will accept Tampa Bay area volunteers to participate in the KidCOVE study. The participants will be separated into two groups. Three out of four children will receive two injections of the vaccine spaced at four weeks apart. The remaining group would receive a placebo made of saline solution.
"The children will receive two shots. They will be randomized three to one so three children will receive the actual vaccine and one child will receive the placebo so there is a 75% chance that your child will be vaccinated with the active vaccine," said Dr. Carina Rodriguez, Professor of Pediatrics and Division Chief, Infectious Diseases for the University of South Florida.
Volunteers who are selected will randomly receive the vaccine or placebo and then be followed by research physicians for 12 months post-vaccination to monitor health and safety.
To participate in the study, volunteers must be:
- Between 6 months and 11-years-old
- In good health or with stable chronic conditions
Volunteers must not:
- Have received an investigational or approved vaccine for COVID-19
- Be currently taking any investigational or approved treatments for COVID-19
- Have tested positive for COVID-19 or been in contact with anyone diagnosed with COVID-19 within 2 weeks prior to vaccine administration
- Have participated in any clinical trial in the last month.
Enrollment begins in the coming days. To enroll, email usfchildrenscovidvaccine@gmail.com or call/text 813-853-1149.